Can the utilization of discarded batteries and greenhouse gases yield a mutually beneficial outcome? Professor Xia Baoyu and his team from the School of Chemistry and Chemical Engineering, HUST have accomplished this feat. Leveraging the recycling of obsolete batteries, they have successfully converted carbon dioxide into economically valuable formic acid, breaking multiple world records. On January 31, the team’s research achievement, titled Durable CO2 Conversion in the proton-exchange membrane system, was published in Nature, a renowned academic journal.

Professor Xia and members of his team
Among more than twenty products obtained from the electrolysis of carbon dioxide, formic acid stands out due to its significant economic value and wide-ranging applications in fields such as fuel cells and pharmaceuticals. Efficient production of high-purity formic acid not only consumes carbon dioxide but also generates substantial economic benefits. Professor Xia and his team have been dedicated to the research for nearly five years, during which they have developed a proton-exchange membrane system for carbon dioxide electrolysis. This system achieves a formic acid faradic efficiency of over 93% and a durable stability of more than 5,000 hours, breaking world records across multiple metrics.
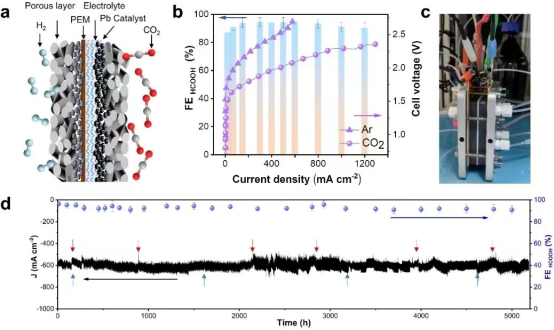
Schematic diagram of CO2 electrolytic system and the performance of CO2 reduction
Why is the research data of the team “far ahead”? In contrast to many other studies, the team employed an acidic electrolyte to complete this system. In the current field of electrocatalytic carbon dioxide reduction technology, researchers usually use alkaline electrolytes. However, a significant portion of carbon dioxide is absorbed by alkaline electrolytes, leading to the generation of substantial carbonate deposits on the electrode surface. This substantially diminishes conversion efficiency and system lifespan.
“The use of acidic electrolyte can remarkably enhance conversion efficiency.” This is an innovative solution proposed by the team. However, many catalysts are susceptible to acid corrosion, making it challenging to carry out reduction reactions efficiently and stably. During the study, Fang Wensheng, a key member of the team, persistently experimented and ingeniously utilized recycled batteries to develop a lead-based carbon dioxide reduction electrocatalyst resistant to acid corrosion.

The novel reaction mechanism allows carbon dioxide to generate only formic acid and a small amount of hydrogen during the electrolysis process while achieving extremely high conversion efficiency. Moreover, this high-performance catalyst can achieve production in terms of kilograms and even tons to meet the demands of industrial application.
During the actual operational process, the team encountered a new challenge: the stability of the electrolysis system fell short of the requirements for industrial application. Through in-depth research, the team identified that the by-product produced in the oxidation reaction of water at the anode corrodes the critical component of proton exchange membrane in the CO2 electrolysis system and impacts the overall performance and lifespan of the entire electrolytic system.

Years of research in the field of energy chemistry inspired Professor Xia to conceive the idea of replacing water with hydrogen. This approach effectively avoided corrosion of the proton exchange membrane and significantly improved stability and lifespan. Additionally, it greatly reduced the power consumption of the system, yielding unexpected positive results for the team.
After overcoming various challenges, the team optimized the design of the reaction device, ultimately achieving low-energy and high-efficiency electrolysis reactions at 3.6 V cell voltage with a current of 20 A. The system has demonstrated the capability to operate continuously for over 5,000 hours.

“This technique has considerable economic value,” said Professor Xia. The carbon dioxide electrolysis reactor designed by the team can achieve a 40-fold increase in area and output while operating efficiently and stably. Based on current market cost estimates, each electrolysis process producing 1 tonne of formic acid is expected to yield a profit of $244, making industrial application feasible.
Written by: Wensheng Fang
Edited by: Chang Wen, Peng Yumeng
