On May 3rd, the latest research result of Professor Pang Yuanjie from the School of Optical and Electronic Information (SOEI) of Huazhong University of Science and Technology, "Constrained C2 adsorbate orientation enables CO-to-acetate electroreduction", was published in Nature. Dr. Jin Jian, Ph.D. student Min Qiuhong from SOEI, Josh Wicks from University of Toronto, and Professor Li Jun from Shanghai Jiao Tong University are the first authors. Professor Pang Yuanjie, Professor E. H. Sargent from the University of Toronto, and Professor Mai Liqiang from Wuhan University of Technology are the corresponding authors of the paper. Huazhong University of Science and Technology is the first affiliation of this paper.
Achieving "peak carbon dioxide emissions" and "carbon neutrality" is the inherent requirement of China's sustainable development and high-quality development, and it is also the inevitable choice to promote the construction of a community with a shared future for mankind. From the perspective of resources and energy development strategy, it is of great and far-reaching strategic significance to use low-grade renewable electric energy to convert carbon dioxide into high value-added carbon-based fuels or chemicals by means of the electrochemical reduction of carbon dioxide, which will protect the environment and promote the sustainable development of society and economy.
This paper reports a new dilute alloy catalyst, which can efficiently convert carbon monoxide to acetic acid under a high-pressure reaction condition. The highest selectivity (Faraday efficiency) of the reaction is 91%, which is similar to the selectivity of carbon-dioxide-to-carbon-monoxide electroreduction, the precursive step of the carbon monoxide upgrade in a cascade carbon dioxide electroreduction. The energy conversion efficiency reaches 34%, double of the existing record. The long-term operation can run for more than 820 hours (with a selectivity of about 80%), which also makes a world record.
In the electrocatalytic reduction of carbon dioxide, the copper-based catalyst is the only catalyst known to have good synthesis efficiency of multi-carbon products, because it can effectively catalyze the carbon-carbon coupling (CC-coupling) step. The sophisticated post-CC-coupling reaction pathways branch toward more than a dozen carbon products, each with a low selectivity. For acetic acid products, the key intermediate has a "monodentate" orientation (*C=C=O) with only one carbon atom bound, and thus "standing" on the copper surface. In this work, a Cu-Ag dilute alloy catalyst was designed, with Cu sites as small as 2-4 atoms each, forcing carbon groups to couple and enter a "monodentate" adsorption state, and efficiently introducing the reaction into the acetic acid generation path. However, small copper sites will face the problem of the inefficient CC-coupling step. Density functional theory computations show that CC-coupling requires higher reactant molecular coverage than usual. Using the advanced in-situ Raman spectroscopy system of the Nano Key Laboratory of Wuhan University of Technology, it is proved that an intermediate in the process of CO reduction is C=C=O or (OH)C=COH configuration. Therefore, in this work, a high-pressure and strong three-phase interface reaction device was designed and granted a national invention patent, which can keep the vapor–liquid equilibrium under high air pressure and work stably, thus meeting the requirement of the molecular coverage of reactants on the catalyst surface. Copper-silver dilute alloy catalyst can convert carbon monoxide into acetic acid with the highest selectivity of 91% in a flow reaction cell with a 5 M potassium hydroxide electrolyte under a pressure of 10 atmospheres and at a full cell voltage of 3.1 volts. The reaction is stable for more than 820 hours with a selectivity of more than 80% and at a full cell voltage of 2.2 volts. The high selectivity and low voltage of the reaction ensure an energy conversion efficiency as high as 34%. The economic and technical feasibility analysis shows that cascade carbon dioxide electroreduction has low cost in the acetic acid synthesis, which ensures the application prospect of this technology in the future.
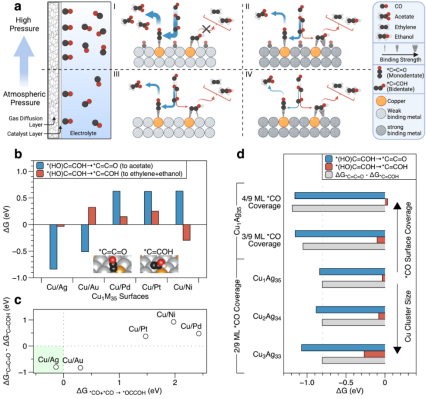
Figure 1. Theory shows that the Cu-Ag dilute alloy catalyst’s surface is more selective for the formation of the monodentically adsorbed *C=C=O intermediate, and it needs a higher coverage of CO surface to ensure the efficient occurrence of carbon-carbon coupling.
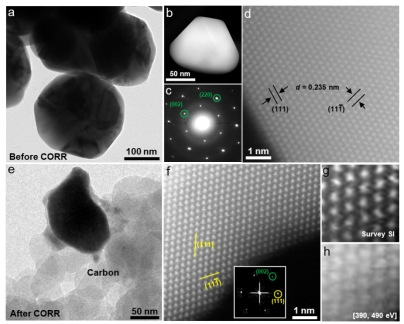
Fig. 2. The TEM characterization of the Cu-Ag dilute alloy catalyst, in which only the lattice of silver is observed, indicating that the Cu element is atomistically dispersed in atomic clusters.
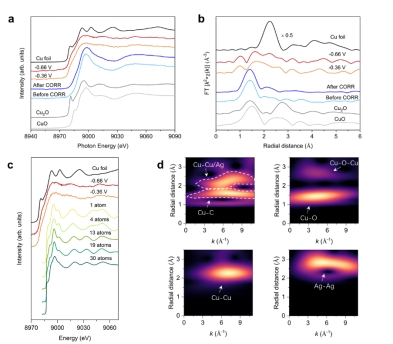
Fig. 3. The synchrotron radiation in-situ characterization of the copper-silver dilute alloy catalyst, further exploring the dispersion state of copper in dilute alloys: the size of each copper cluster is about 2-4 atoms.
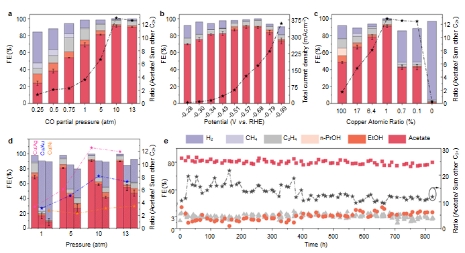
Fig. 4. The Cu-Ag dilute alloy catalyst’s performance in high-pressure reaction devices: In a flow reaction cell under 10 atmospheres of pressure, the selectivity of the acetic acid electrosynthesis can reach 91%; in the reaction cell of the membrane electrode assembly under 10 atmospheres of pressure, the selectivity of the acetic acid electrosynthesis can reach 85%, and the operation can run for more than 820 hours with a selectivity of about 80%.
To sum up, this work has realized a sustainable, cascade process to convert carbon dioxide into acetic acid by using low-grade clean electric energy with high selectivity and high energy conversion efficiency. This work has confirmed the application potential of the carbon dioxide electrolysis technology in distributed clean energy storage, and the feasibility of the green synthesis of carbon-based chemicals using the carbon dioxide electrocatalytic conversion technology, which will contribute to the achievement of the "dual carbon goals".
Professor Pang Yuanjie was selected into the national talent plan in 2018, and his research direction is electrocatalytic carbon dioxide reduction. He is committed to developing a high-pressure three-phase interface reaction system, completing the efficient transformation of carbon dioxide into multi-carbon chemicals, building a high-pressure-compatible operando characterization technique to establish a pressure-related structure-effect correlation of electrocatalysts, and developing novel, highly stable and efficient technical demonstration for carbon dioxide electroreduction.
Paper link: https://www.nature.com/articles/s41586-023-05918-8
Source: School of Optical and Electronic Information
Edited by: Chang Wen, Peng Yumeng
